Our primary focus is to develop completely novel immuno-oncology (IO) therapeutics that each target a specific BTN(L) to engage immune cells against solid tumors and hematologic malignancies. ICT01, an anti-BTN3A mAb that activates γ9δ2 T cells, a subpopulation of unexploited killer lymphocytes, has entered into clinical development in 2020. Follow-on programs targeting BTN2A and BTNL3/BTNL8 are currently in the discovery phase but demonstrate potential to modulate TAMs and δ1 T cells, respectively.
With a broad range of development programs that target both innate and adaptive immunity, ImCheck Therapeutics aims to extend the benefits of effective immunotherapy to more patients.
Butyrophilins
Immune modulation beyond the B7/CD28 superfamily
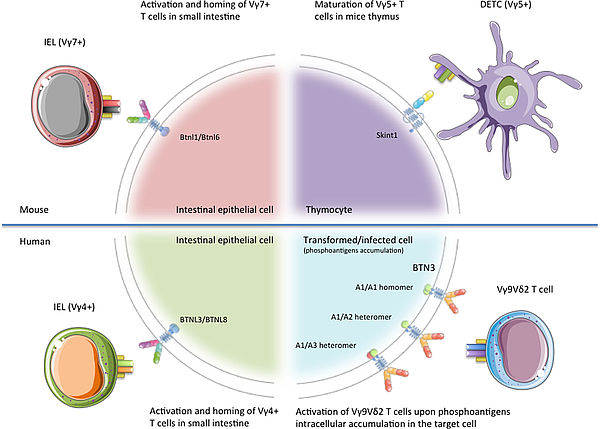
Work in Prof. Daniel Olive’s laboratory largely contributed to the discovery and understanding of the immunoregulatory properties of the BTN(L)s. Today, ImCheck Therapeutics is developing a series of programs to target different members of this family, of which the most advanced target is BTN3A, a key activator of γ9δ2 T cells. Importantly, the 3 isoforms of BTN3A are overexpressed in various solid and hematological cancers, as well as on the surface of multiple immune cell populations.
ImCheck is maximizing its drug development pipeline by generating mAbs to each member of the BTN(L) superfamily to activate or inhibit different immune cell subsets as a therapeutic approach in immuno-oncology (IO), infectious diseases, and autoimmune disease (AID), respectively.
Illustration from New Insights Into the Regulation of γδ T Cells by BTN3A and Other BTN/BTNL in Tumor Immunity, Blazquez et al. Front Immunol 2018 11;9;1601