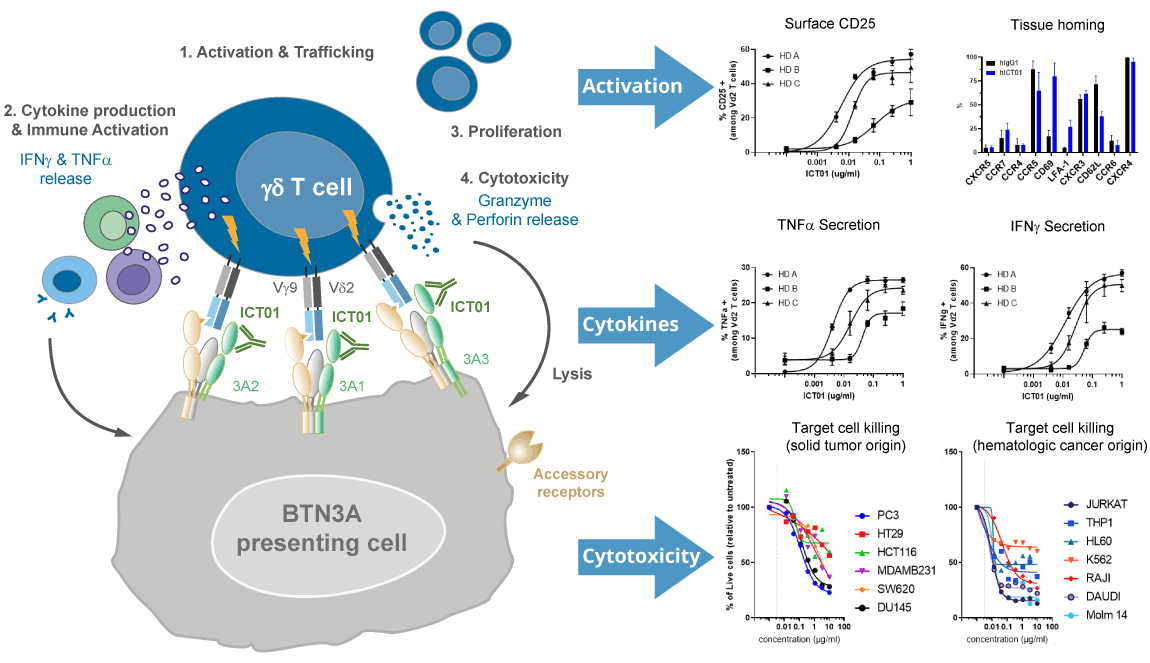
ICT01 is a humanized, anti-BTN3A (also known as CD277) monoclonal antibody that selectively activates γ9δ2 T cells, which are responsible for immunosurveillance of malignancy and infections. The three isoforms of BTN3A targeted by ICT01 are overexpressed on many solid tumors (e.g., melanoma, urothelial cell, colorectal, ovarian, pancreatic, and lung cancer) and hematologic malignancies (e.g., leukemia and lymphomas) and also expressed on the surface of innate (e.g., γδ T cells and NK cells) and adaptive immune cells (T cells and B cells). BTN3A is essential for the activation of the anti-tumor immune response of γ9δ2 T cells.
As demonstrated by data presented at past AACR, ASCO, ASH, ESMO and SITC conferences, ICT01 selectively activates circulating γ9δ2 T cells leading to migration of γ9δ2 T cells out of the circulation and into the tumor tissue and triggers a downstream immunological cascade through secretion of pro-inflammatory cytokines, including but not limited to IFNγ and TNFα, further augmenting the anti-tumor immune response. Anti-tumor activity and efficacy of ICT01 have been shown in patients across several cancer indications.
In July 2025, ICT01 was granted Orphan Drug Designation by both the FDA in the United States and the EMA in Europe for the treatment of acute myeloid leukemia (AML).
Mechanism of action