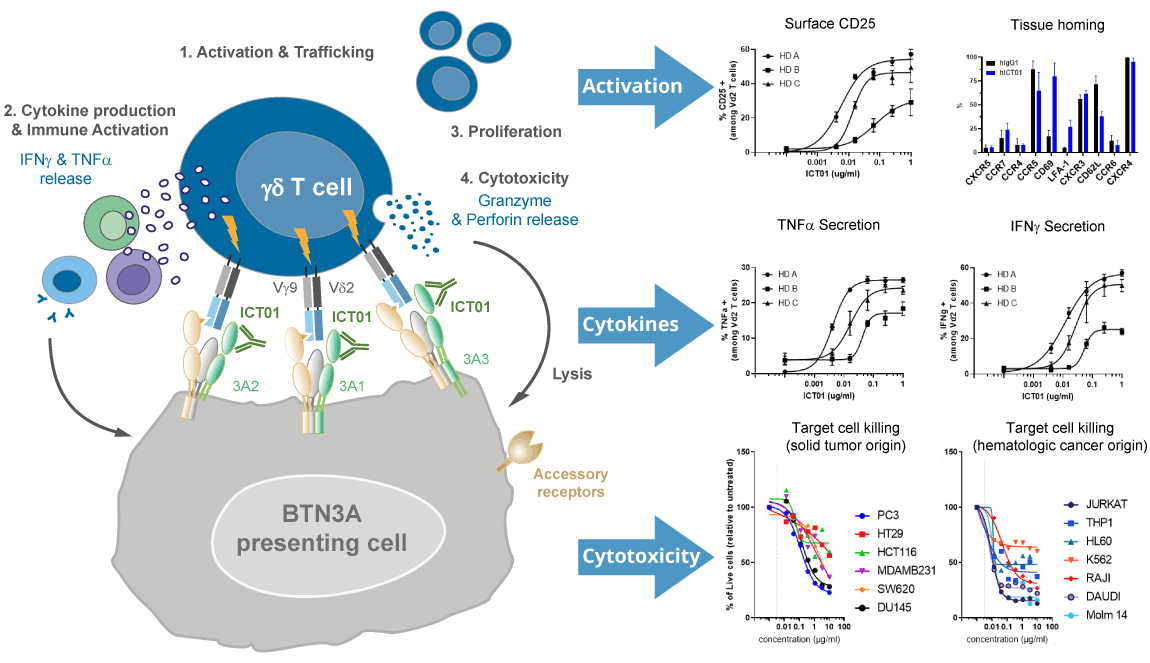
ICT01 is a humanized, anti-BTN3A (also known as CD277) monoclonal antibody that selectively activates γ9δ2 T cells, which are part of the innate immune system that is responsible for immunosurveillance of malignancy and infections. The 3 isoforms of BTN3A targeted by ICT01 are overexpressed on a number of solid tumors (e.g., bladder, colorectal, melanoma, ovarian, pancreatic, lung) and hematologic cancers (e.g., leukemia & lymphoma) and also expressed on the surface of innate (e.g., γδ T cells and NK cells) and adaptive immune cells (T cells and B cells). BTN3A is essential for the activation of the anti-tumor immune response of γ9δ2 T cells.
ImCheck has presented encouraging results from the ongoing EVICTION trial in oral presentations at major medical conferences since 2021, including AACR, ESMO and SITC, demonstrating the safety of ICT01 and its potent mechanism of action leading to activation, migration and tumor infiltration of circulating γ9δ2 T cells, CD8 T cells & NK cells and initial tumor responses.
Mechanism of action